What is C–H Functionalization?
Synthetic organic chemistry is one of the key enabling sciences. The main ethos behind the field is to take the simple organic chemical building blocks available from natural resources, such as from petroleum or plant biomass, and transform them into chemicals of high value. Many sectors, both academic and industrial, rely on this process. It is used in biology to make small molecule probes, in medicine to make pharmaceuticals and in materials science to make the building blocks of plastics to name but a few.
The most basic organic molecules are made up from carbon and hydrogen atoms. These carbon-based frameworks are typically considered to be the inert, unreactive ‘backbone’ of an organic molecule because of the large amounts of energy required to make and break these bonds. Conventional synthetic organic chemistry relies upon ‘functional groups’, an atom, or group of atoms, that replaces hydrogen, typically with oxygen (O), nitrogen (N), boron (B), sulfur (S) or halogen (X) atoms. These functional groups can then be modified to transform the organic molecules, through a series of steps or reactions, into high-value complex molecules. The greater the complexity of the desired product, the greater the number of reactions required to synthesize it (Shown Left: a functional group (represented by the red atoms), is introduced into a simple hydrocarbon molecule as a method to construct a more complex, useful molecule).
Based on this mode of thinking, synthetic organic chemistry has made remarkable advances in the past 100 years. We can now construct molecules of exquisite complexity in an elegant and practical fashion, facilitating the advance of modern medicine and manufacturing. However, reliance on functional groups for performing a reaction comes with a price. Functional groups, when found in Nature’s molecules are often not in the desired location and as such either require introduction or modification before subsequent conversion to the desired product. Furthermore, upon completion of the desired reaction, removal of these reactive species and their corresponding potentially harmful by-products can be difficult, time-consuming and expensive.
Carbon–Hydrogen (C–H) functionalization fundamentally challenges this mode of operation; what if we no longer rely on functional groups for reactions and instead use C–H bonds as reactive partners? This has two immediate benefits: C–H bonds are ubiquitous in organic molecules, so structural modifications can take place at a wide variety of position and the amount of waste is significantly reduced, with no potentially harmful byproducts formed from the presence and reaction of the volatile functional groups. The benefits to industry and manufacturing would be immense, streamlining many processes and facilitating a sustainable approach.
Sounds too good to be true? The C–H bond was first observed to be a reaction partner in the early 1960’s, but it has only been in the past 10-15 years that the real potential of this approach has begun to be developed. The reason for the slow uptake in an approach that offers such promise is perhaps the central challenge to all organic chemistry: Selectivity. With functional groups you can select between different functional groups thanks to the variety of atoms and the difference in possible bond strengths. On the other hand, C–H bonds are ubiquitous in organic molecules, which often contain multiple C–H bonds that are energetically similar or equivalent. Thus the main challenge in this field has been to develop techniques that provide the required control and can reliably select specific sites on the basis of small energetic or spatial bias.
“The successful implementation of C–H functionalization is a multifaceted problem requiring the engagement and communication of scientists with different skill sets,” says Professor Huw Davies, Asa Griggs Candler Professor of Chemistry at Emory University. “For decades the chemistry was primarily explored by inorganic organometallic chemists and thus the broader challenges and opportunities as it relates to organic synthesis was not the central focus.”
The advent of transition-metal catalysis has made this methodology possible. By employing metals such as rhodium, ruthenium, palladium and iridium, the highly reactive chemicals required to react with C–H bonds can be mediated and controlled. Many innovative solutions have been developed to control which C–H bond is selected, but they can all fall into three general classes:
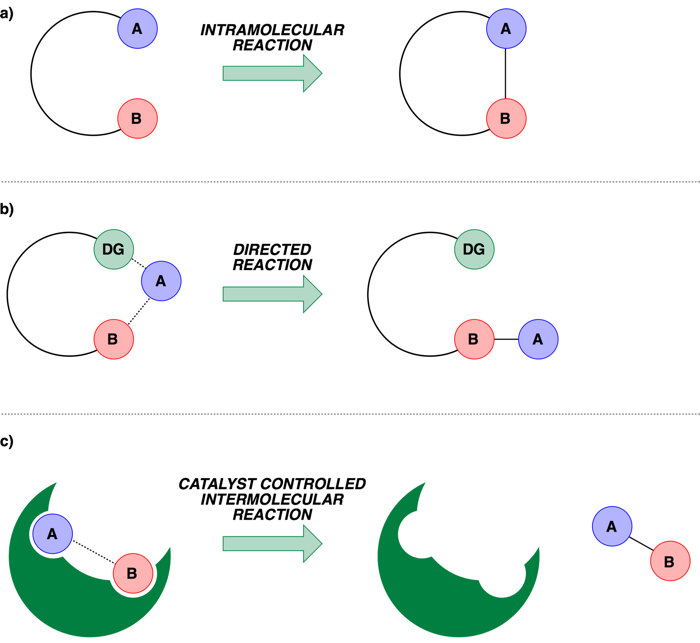
The earliest method employed to assert control was to use intra-molecular systems, that is to have the two reactive sites in the same molecule and this would pre-orientate the species in a particular spatial conformation to bias the outcome (a). Building upon this idea, arguably one of the most successful approaches to date employs what is termed a ‘Directing Group’. A directing group is a functional group that can temporarily bind to the transition-metal catalyst and direct the site of reaction to an adjacent or nearby C–H bond, asserting control through proximity (b, in this case the directing group is represented by DG). The third general class uses the shape of a catalyst to control how the two reactive sites approach each other, which requires sophisticated design of the environment that surrounds the catalyst (c, the catalyst environment is represented by the green shape, analogous to the active site of an enzyme).
Using either one of these strategies, or more recently a combination thereof, researchers from across the globe have developed methods for the selective conversion of C–H into C–C, C–O, C–N, C–B and C–X (where X = F, Cl, Br, I) bonds. The scope is broad, with both aromatic (sp2) and aliphatic (sp3) C–H bonds found to be suitable reaction partners. These transformations are being refined to display exquisite levels of selectivity. In addition, the methods have been demonstrated to be powerful and robust, exhibited in the synthesis of numerous products found in nature and drug-like molecules.
However, there is much that remains to be done in this field and this is the premise for the NSF-funded Center for Chemical Innovation (CCI), the Center for Selective C–H Functionalization (CCHF). The CCI’s bring together researchers from across the USA to address the big challenges that face chemistry. The goal of the CCHF is to bring C–H functionalization into the main stream of synthetic organic chemistry. In order to do this a number of challenges must be met:
Building a ‘toolbox’ of catalysts to selectively functionalize a wide range of C–H bonds and incorporate more earth abundant metals, such as iron, nickel and cobalt, removing the reliance on rare-metals.
Expand the scope of transformations and possible substrates.
Demonstrate the potential of this methodology in the streamlined synthesis of molecules of importance.
Address the issues associated with scaling up this chemistry for industrial application.